anti ige xolair
Develop highly selective and sensitive PK and ADA assays for the monoclonal antibody drug omalizumab Xolair using our range of ready-made anti-idiotypic and complex-specific antibodies. It binds specifically to the Cepsilon3 domain of immunoglobulin E IgE.

Xolair Allergic Asthma Treatment Steps Xolair Omalizumab
Xolair binds the Cε3 domain of IgEs thus inducing a con-formational change of the immunoglobulin and provoking a concealment of FcRI and FcεεRII receptors binding sites thus precluding binding by IgE Xolair binds only free and not bound human IgEs.

. Omalizumab selectively binds to the Cϵ3 domain of IgE at the site of FcϵR1 binding thus blocking binding of IgE to effector cells. Allergic rhinitis is one of the most common and increasing diseases worldwide affecting more than 500 million people. Xolair Omalizumab wordt ook wel Anti-IgE genoemd.
Xolair therapie Dit is een medicijn voor de behandeling van ernstig allergisch astma. Xolair prevents IgE from turning on inflammatory cells called mast cells and basophils. Isotype and Serum Controls.
Monoclonal anti-human IgE administration on the accuracy of serological immunoassays for IgE has not been reported. Normally excess IgE antibodies attach to mast cells and bind to allergens causing the release of histamine and other chemicals into the bloodstream. Omalizumab sold under the brand name Xolair is a medication used to treat asthma nasal polyps and urticaria hives.
The most common adverse reactions 2 xolair-treated patients and more frequent than in placebo for xolair 150 mg and 300 mg respectively included. COVID-19 omalizumab and other anti IgE drugs Omalizumab is a recombinant human anti-IgE antibody originally designed to reduce sensitivity to allergens and blocks IgE binding to the high-affinity IgE receptor FcεRI. XOLAIR omalizumab is indicated for.
Randomized trial of omalizumab anti-IgE for asthma in inner-city children. Omalizumab Xolair is the anti-IgE medicine now available. Omalizumab anti-IgE monoclonal antibody E25 E25 humanised anti-IgE MAb IGE 025 monoclonal antibody E25 olizumab rhuMAb-E25 Xolair is a chimeric monoclonal antibody.
The effect of Xolair Omalizumab. Omalizumab is used to treat IgE-mediated diseases such as chronic idiopathic urticaria CIU and moderate to severe allergic asthma. Adults and pediatric patients 6 years of age and older with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and whose symptoms are inadequately controlled with.
It also inhibits FcεRI -associated with activation in mast cells by removing surface IgE. Highly specific to omalizumab or the omalizumab-hIgE complex Fully human surrogate positive control or calibrator. Custom peptides Antibodies.
The bond of the anti-IgE prevents basophils and mast cells de-. N Engl J Med 2011. Xolair had worldwide sales of US835 million in 2016.
Anaphylaxis presenting as bronchospasm hypotension syncope urticaria andor angioedema of the throat or tongue has been reported to occur after administration of XOLAIR. Xolair is made to be similar to natural antibodies and is designed specifically to capture most of the IgE and block the allergic response. But the mechanism of action lends itself to treating allergic rhinitis.
Omalizumab Xolair is a recombinant humanized monoclonal antibody that selectively binds to human immunoglobulin E IgE. Headache 12 6 nasopharyngitis 9 7 arthralgia 3 3 viral upper respiratory infection 2 1 nausea 1 3 sinusitis 1 5 upper respiratory tract infection 1 3 and. Cepsilon3 is the site of high-affinity IgE receptor binding.
Anaphylaxis has occurred as early as after the first dose of XOLAIR but also has occurred beyond 1 year after beginning regularly administered treatment. Removal of circulating free IgE by the recombinant humanized monoclonal anti-IgE antibody omalizumab Xolair represents a novel therapeutic approach. Busse WW Morgan WJ Gergen PJ et al.
Xolair works by blocking IgE. Xolair is a monoclonal anti-IgE antibody that binds to IgE antibodies in the bloodstream. Toon meer Afdeling Longziekten.
This reduces symptoms such as wheezing coughing swelling itching and runny nose. En om de hoeveelheid medicijnen die dagelijks gebruikt worden iets te kunnen verlagen. XOLAIR is given in 1 or more injections under the skin subcutaneous 1 time every 2 or 4 weeks.
The patents on Xolair will expire in the US on 20 June 2017 and in Europe in August 2017 1. The originator product Novartis Xolair omalizumab was approved by the US Food and Drug Administration FDA on 20 June 2003 and by the European Medicines Agency EMA on 25 October 2005 1. The size of the disease relevant IgE antibody fraction in relation to total-IgE predicts the efficacy of anti-IgE Xolair treatment.
The anti-IgE Xolair treatment reduces the asthma frequency also it improved the patient life quality by inducing positive effects on symp. Het doel van Xolair therapie is om de klachten van astma beter onder controle te krijgen. In people with asthma and nasal polyps a blood test for a substance called IgE must be performed before starting XOLAIR to determine the appropriate dose and dosing frequency.
Have incomplete control of moderate to severe persistent asthma. Xolair is approved by the FDA for use with patients 6 years of age and older who. Omalizumab is a recombinant DNA-derived humanized IgG1k monoclonal antibody that specifically binds to free human immunoglobulin E IgE in the blood and interstitial fluid and to membrane-bound form of IgE mIgE on the surface of mIgE.
Currently Xolair which is an anti-IgE antibody is indicated for moderate to severe persistent asthma and chronic idiopathic urticaria. 23 Xolair is a monoclonal antibody made using biotechnology. Jan 09 2020 Data show ligelizumab binds to immunoglobulin E IgE a key driver of chronic spontaneous urticaria CSU with significantly higher affinity than current standard of care Xolair omalizumab1 The study published in Nature Communications suggests ligelizumab has the potential to be more effective than Xolair in treating CSU.
1 IgE is an antibody that is responsible for many allergy symptoms. Johansson SG Nopp A Oman H et al.

From Ige To Omalizumab The Journal Of Immunology
Getting Your Xolair Injection For Allergic Asthma Xolair Omalizumab

Ligelizumab Qge031 Vs Omalizumab Xolair Los Angeles Allergist

Anti Immunoglobulin E Therapy World Allergy Organization Journal

Xolair Omalizumab Uses Dosage Side Effects Interactions Warning
Xolair Fda Prescribing Information Side Effects And Uses

Refractory Asthma Mechanisms Targets And Therapy Cough Treatment Asthma Cure Asthma

What Is Xolair Allergic Asthma Treatment Xolair Omalizumab
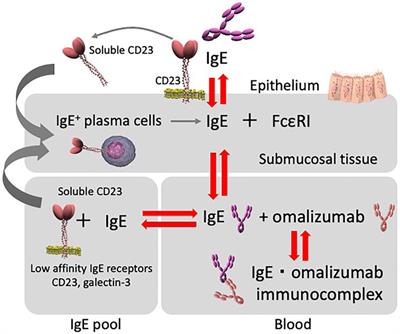
Frontiers Omalizumab And Ige In The Control Of Severe Allergic Asthma Pharmacology

Mechanisms Of Inhibitory Effect Of Omalizumab On Allergic Inflammation Download Scientific Diagram

Management Of Severe Asthma An Update 2014 Severe Asthma Asthma Chronic Sinusitis

Pediatric Usage Of Omalizumab A Promising One World Allergy Organization Journal

Omalizumab Mechanism Of Action Uptodate

Omalizumab Safety In Pregnancy Journal Of Allergy And Clinical Immunology

Xolair Omalizumab Side Effects Important Safety Information

Mechanism Of Action Of Omalizumab Omalizumab Binds To Ige Thus Download Scientific Diagram


Comments
Post a Comment